
Fixed on Nitrogen
One of the most common elements in the universe, nitrogen is also a crucial ingredient in fertilizer. All living things need it to survive. But it can kill, too.
By Elliot Tritto

ear the winding Mississippi River in southeastern Louisiana, CF Industries’ 1,400-acre Donaldsonville Complex rises in a steam-punk spectacle. The behemoth of cooling towers, tanks, furnaces, pipes and smokestacks breathes out steam – and sometimes the ammonia leaks that can result in a shelter-in-place order for the local community.
At night, the colossal complex looks like a small city. A vast array of bright lights guides workers in a busy hive of chemical manufacturing. Nearly 1,000 workers at the plant help produce nearly 8 million tons of nitrogen products a year for agriculture and other industries. Ammonia, ammonium nitrate, urea and diesel exhaust fluid ship from the complex via pipelines, rail, truck and river and ocean barges.
CF’s Donaldsonville operation is the largest ammonia plant in the world, according to the company. Ammonia, composed of three hydrogen atoms bonded to a nitrogen atom, is part of the process of synthesizing nitrogen. Among the “Big 3” nutrients in commercial fertilizers – nitrogen, phosphorus and potassium – nitrogen is crucial for building the proteins that in turn build the tissues in most living things.
One of the most common chemical elements, nitrogen makes up 78% of the air. Plants, animals and people can’t live without it. But they also can’t use it in its gas form. It needs to be “fixed.” The fix happens slowly in nature as plants and microorganisms convert atmospheric nitrogen into ammonia. For the past century, the fix has also been super-charged for industrial purposes in the so-called Haber-Bosch process that combines nitrogen from the air with hydrogen under extremely high pressure to create ammonia.
In 1918, the process won the German chemist Fritz Haber a Nobel Prize in Chemistry for his “exceedingly important means of improving the standards of agriculture and the well-being of mankind.” A century later, demographers estimate only half the 8 billion people now living on the planet could be fed without synthetic nitrogen fertilizers.
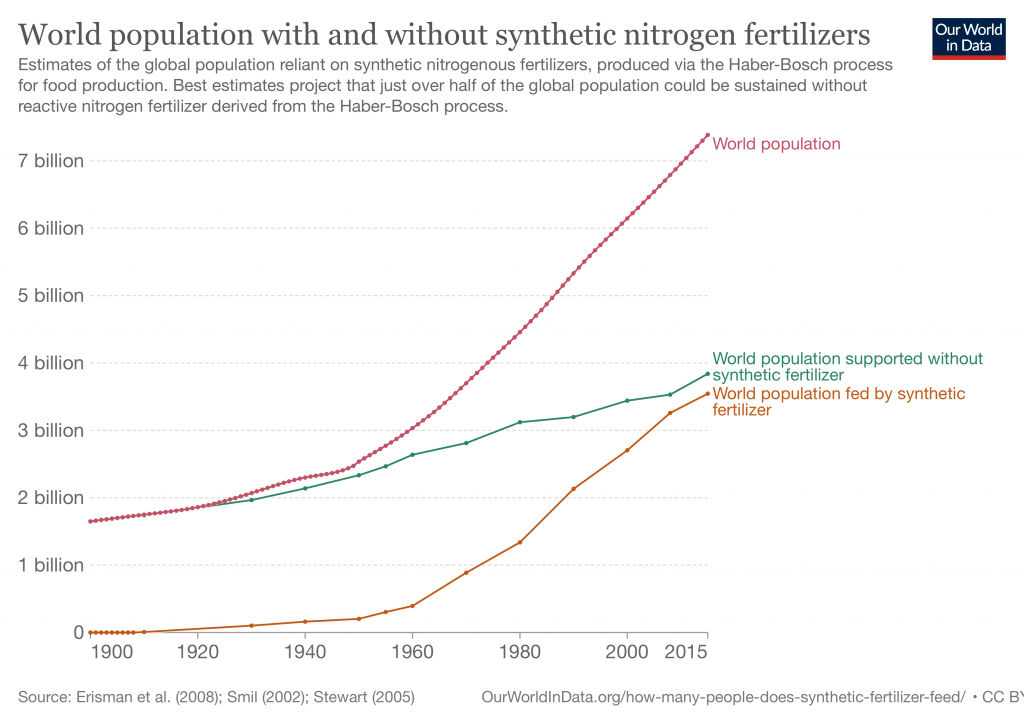
But the same nutrient that feeds billions of people can also kill, in chemical weapons, in factory explosions or via overdoses in the environment. Over-applied on farms, excess nitrogen runs off into waterways where it fuels algae that proliferate, block light and starve other plants and animals of oxygen.
“There is no question that nitrogen fertilizer provides a tremendous benefit to society by boosting the production of food to feed billions of people around the world, including in developing nations,” the Environmental Integrity Project reported this spring in a call for greater regulation of the industry due to threats to public safety and the environment. “But the over-application of chemical fertilizers also carries a cost for the environment, and that harm increases when the manufacture of chemicals is poorly regulated.”
An invention that changed the world
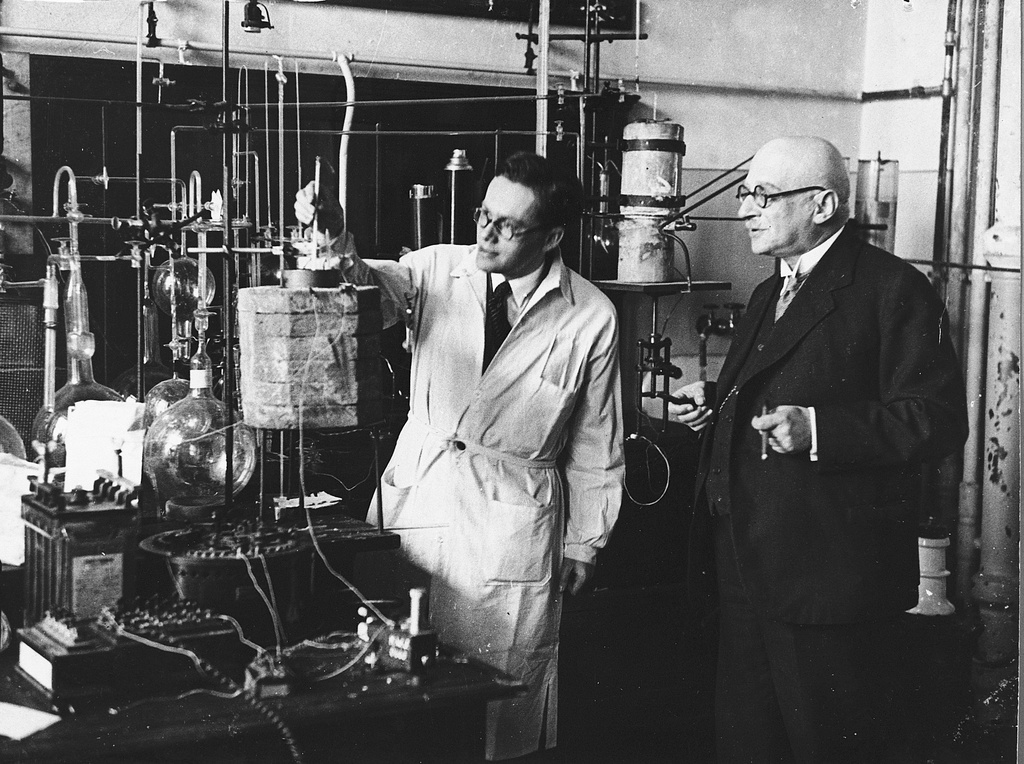
In the early 20th century, farmers in Europe and the United States relied on imports like saltpeter from Chile and huge mountains of guano from Peru as a source of fixed nitrogen. Haber’s discovery of synthetic ammonia production changed the world, said the physicist Benjamin Johnson at the Max Planck Institute for the History of Science in Berlin.
A major energy transition, at that time to fossil fuels, was underway. Agriculture was industrializing. People were excited about Haber’s invention but the hard part was scaling it up, said Johnson, author of the 2022 book Making Ammonia: Fritz Haber, Walther Nernst, and the Nature of Scientific Discovery.
The same year he patented his discovery, in 1908, the chemical company BASF contracted with Haber to develop an industrial-scale, high-pressure synthesis of ammonia. BASF chemical engineer Carl Bosch was assigned to the project. He had to build the plant and mechanics that could stand intense gas pressure and temperatures. Bosch “had to come up with some special casings to keep this gas mixture under pressure and keep it sealed so that the pressure ovens wouldn’t break,” said Johnson.

At first, Bosch’s tubes would burst, even when made of carbon steel. Under high pressure and above 400◦ C, most of the materials he tried inevitably disintegrated. He experimented with different types of steel and catalysts and eventually settled on the industrial process still used at CF industries and other plants to produce ammonia from nitrogen and hydrogen. Unveiled in 1914, Bosch’s machine was 26 feet tall and could make 198 pounds of ammonia an hour. With annual production at 4.3 million tons, CF Industries’ Donaldsonville plant now operates around the clock to produce 495 tons of ammonia per hour.
In 1931, Bosch, like Haber before him, won the Nobel Prize in Chemistry for his contribution to a better world.
Father of modern farming and chemical warfare

The Haber-Bosch process revolutionized farming, doubling the number of people that one acre of land could feed.
But making nitrogen compounds from the air for fertilizer started Fritz Haber down a dark path to chemical warfare. Early in World War I, Germany faced a munitions crisis. Haber, by then director of the Kaiser Wilhelm Institute for Physical Chemistry in Berlin, set out to help. He coaxed military and industrial leaders, scientists and politicians to develop large-scale chemical weapons production, according to the German historian Margit Szöllösi-Janze.
Haber orchestrated the first wartime use of poison gas at the Western Front at Ypres, Belgium, in April 1915, working with meteorologists to catch prevailing winds. He led the German Army to plant 6,000 steel cylinders in trenches. When soldiers opened the valves, 160 tons of chlorine gas floated downwind. The new weapon killed more than 1,000 French and Algerian soldiers within minutes and wounded thousands more.
Much more than those casualties, Haber was a catalyst for the new, global chemical warfare that saw other countries racing to develop explosives and chemical weapons, according to Szöllösi-Janze. By the end of the war, an estimated 1,000 scientists had worked on Germany’s poison gas program.
Haber was celebrated for his “success” at Ypres and promoted in a gathering at his director’s mansion on the evening of May 1, 1915. The same night, his wife, the PhD chemist Clara Immerwahr Haber, committed suicide by shooting herself with her husband’s army pistol. Historians disagree about whether her suicide was prompted by dismay over her husband’s involvement in mass chemical weapons.
Haber’s legacy is that of a “scientist who has been praised as a benefactor of mankind, and, at the same time, accused of being a war criminal,” Szöllösi-Janze wrote. “His scientific work transformed both food production and warfare.”
Fertilizer Boom

A century after the invention of Haber-Bosch, about 30 chemical plants in the United States produce ammonia for the fertilizer industry. That number could grow by another third in the next few years, according to the Environmental Integrity Project (EIP), based on nine proposals to build new ammonia fertilizer plants and another three plant expansions.
In its report The Fertilizer Boom, EIP argues the expansion poses risks for public safety and the environment. Over the last century, 641 people in the United States and 1,237 globally have been killed in major nitrogen fertilizer explosions, according to EIP. The nonprofit is pressing for the Environmental Protection Agency to add ammonium nitrate to the list of more than 140 hazardous chemicals that require manufacturers to plan for disasters and share information with local emergency planners.
The industry also poses risks to the environment by way of water and air pollution and significant greenhouse gas emissions. Researchers publishing in the journal Scientific Reports found that globally, synthetic nitrogen production and use makes up more than 2 percent of greenhouse gas emissions – more than for worldwide passenger and commercial aviation combined.
Nitrogen fertilizer is also a big part of the nutrient runoff implicated in toxic algae outbreaks around the nation and in the Gulf of Mexico’s “dead zone.” Nitrogen also is released directly by fertilizer manufacturing plants. Matt Rota, senior policy director for Healthy Gulf, explained, “if you’re making nitrogen fertilizer, you’re going to be discharging some nitrogen into the water as well, which isn’t preserved in the process.”
Healthy Gulf and the Environmental Integrity Project are among 13 environmental groups that filed a federal lawsuit this spring against the EPA for what they described as outdated regulations on pollution control technology. According to Healthy Gulf, 21 U.S. nitrogen fertilizer plants discharged 7.7 million pounds of nitrogen into waterways in 2021.
In the bigger picture, U.S. farmers apply about 21 million metric tons of fertilizer annually, more than half nitrogen-based. Roughly half of the nutrients are not taken up by crops but instead are lost to runoff, ending up in streams, lakes and rivers. Rota said nitrogen from corn and soybean crops in the Midwest carried by the Mississippi River to the Gulf is a particular problem for the dead zone.
Greener future?
CF Industries did not answer questions related to this story. The company has announced that it is pursuing two new kinds of ammonia production projects that will allow CF to reduce carbon emissions 25% by 2030, and to achieve net-zero carbon emissions by 2050.
Green ammonia means ammonia produced with renewable energy. CF is adding an electrolysis system at Donaldsonville that will allow the plant to produce 20,000 tons of green ammonia a year, just 0.25% of their annual production.

Blue ammonia means ammonia is conventionally produced, but the CO2 emitted in the process is captured and stored away. Last fall, CF announced plans to invest nearly $2 billion on a carbon-capture project at Donaldsonville that will allow the plant to produce 1.7 million tons of blue ammonia per year.
Louisiana Gov. John Bel Edwards praised the investment in helping to make Louisiana a leader in the transition to clean energy. CF Industries president and CEO Tony Will said in a press release with Edwards that the company will be first on the market with a significant volume of blue ammonia. “This will enable us to supply this low-carbon energy source to hard-to-abate industries that increasingly view it as critical to their own decarbonization goals,” Will said.
But there are plenty of critics who say that carbon capture and storage is risky, flawed and unproven technology. Projects such as CF’s will earn large financial incentives under last year’s Inflation Reduction Act. “It looks like more of the same,” said Monique Harden, director of law and policy and community engagement program manager at the Deep South Center for Environmental Justice. CF’s plan would capture emissions at the Donaldsonville plant, transport them about 100 miles, and store them underground on property owned by ExxonMobil.
Harden, who has worked for 20 years as an attorney helping predominantly African American communities win environmental justice victories, said carbon capture and storage projects could pose dangers to citizens who already face health and safety risks living near industrial plants, as well as those in the path of pipelines and near underground storage facilities.

Rota said it’s telling that CF Industries is investing so much more in the blue ammonia that still emits carbon. “I think that shows you two things,” he said. “They are perfectly capable of doing green ammonia, and Louisiana has continued to be looked at as a petrochemical state and a state where it doesn’t matter.”
Johnson, the “Making Ammonia” author, said that “back in Haber’s day, they didn’t care about carbon dioxide but we do now.” So it’s exciting to see new technology that uses electrolysis to make ammonia “green.”
“Our choices as humans about whether we want that technology and whether we want to fund it then becomes very central to the implementation process,” Johnson said.
Haber’s story “illustrates how scientific progress spans generations and adapts to new circumstances,” he wrote in Making Ammonia. “We can learn from these events and apply the lessons.”
This story is part of The Price of Plenty, a special project investigating fertilizer from the University of Florida College of Journalism and Communications and the University of Missouri School of Journalism, supported by the Pulitzer Center’s nationwide Connected Coastlines reporting initiative.
Read Next: Part II: Justice
 The Price of Plenty
The Price of Plenty